



We are a pioneer in the introduction of single-use instruments, continuing the company's long tradition of innovation.
Launched in 2002, Vernacare's Instrapac® range of single-use instruments has been a significant advancement in the healthcare industry. Being a pioneer in the introduction of single-use instruments, the company has continued its tradition of innovation, providing healthcare professionals with a valuable alternative to reusable instruments.
Precision Protection Performance

We have a range of single-use instruments across podiatry, ENT, dental, women's health, and general surgery.
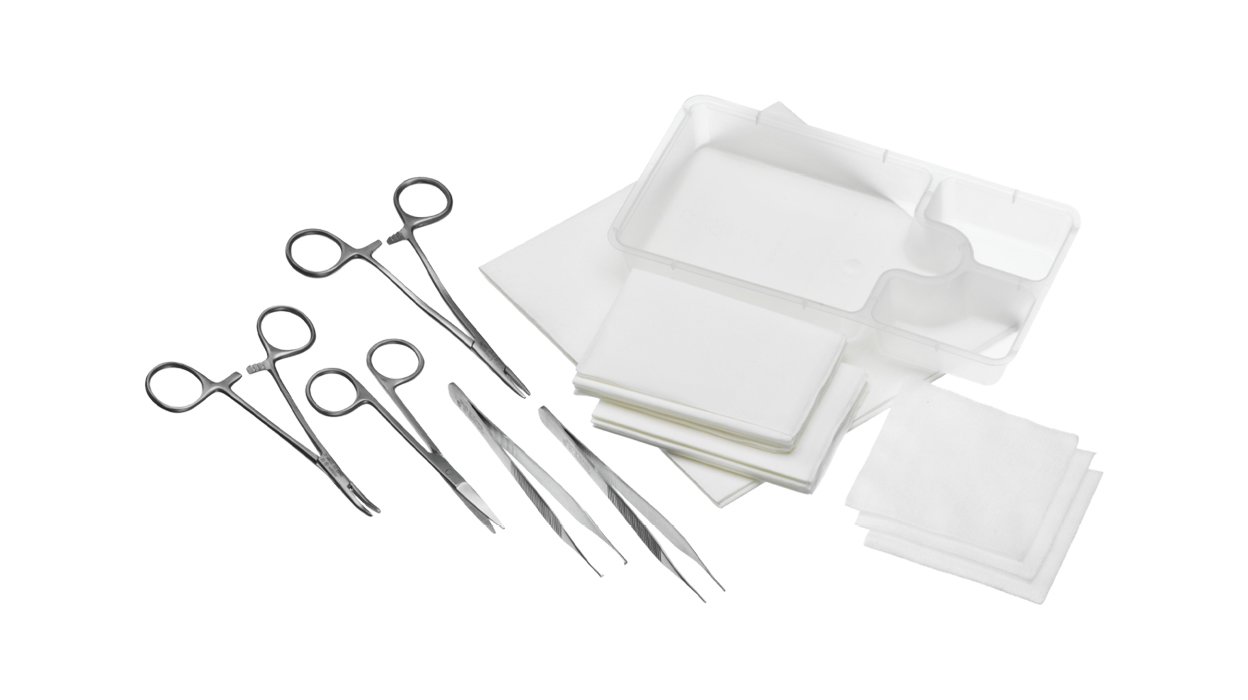
With handy procedure packs, you can access all the sterile products you need in one sterile, pre-packaged solution.

UK Production Facility
Almost all of our products are produced at our large UK manufacturing facility in Worksop, Nottinghamshire. Single-use instruments, which are classed as medical devices, are AQL inspected and hygienically packaged in a large Class 7 cleanroom, then sterilised in Ethylene Oxide gas to meet rigorous quality standards.
Products comply with the relevant British Standards for surgical instruments and the quality control team ensure that products meet specification before being released for packing. All metal products are manufactured using medical grade stainless steel and plastic products are manufactured using biocompatible certified polymers. The Instrapac® range is packaged in high quality, easy to peel packs which feature indicators to verify that the contents are sterile. All procedure packs are presented wrapped in a sterile field for convenience.
Compliance with Legislation and Standards
It is a legal requirement for all medical devices to comply with the Medical Devices Directive (MDD) 93/42 EEC and carry the CE mark. Regulatory requirements differ according to the risk attached to the use of the product. The lower the risk, the easier it is to comply with the directive.
Single-use instruments are relatively high risk as many are surgically invasive and therefore classified as IIa devices. They are therefore subject to greater levels of control than reusables, which are Class I devices, except those used on central nervous tissue. Class I devices can be sold without any authorisation from an independent third party - a notified body. Class IIa devices, which make up a large proportion of the Instrapac® range, require a notified body to assess the production of the device to Annex IV, V or VI of the directive and issue a certificate of conformance before a CE mark can be applied and the product legally sold.
Certification by the British Standards Institution (BSI) verifies that Instrapac® products meet fully the requirements of the regulation.

Find out how we can help you
Come and talk to us about your requirements, partnering with us or find out how we can help you.
Contact us