



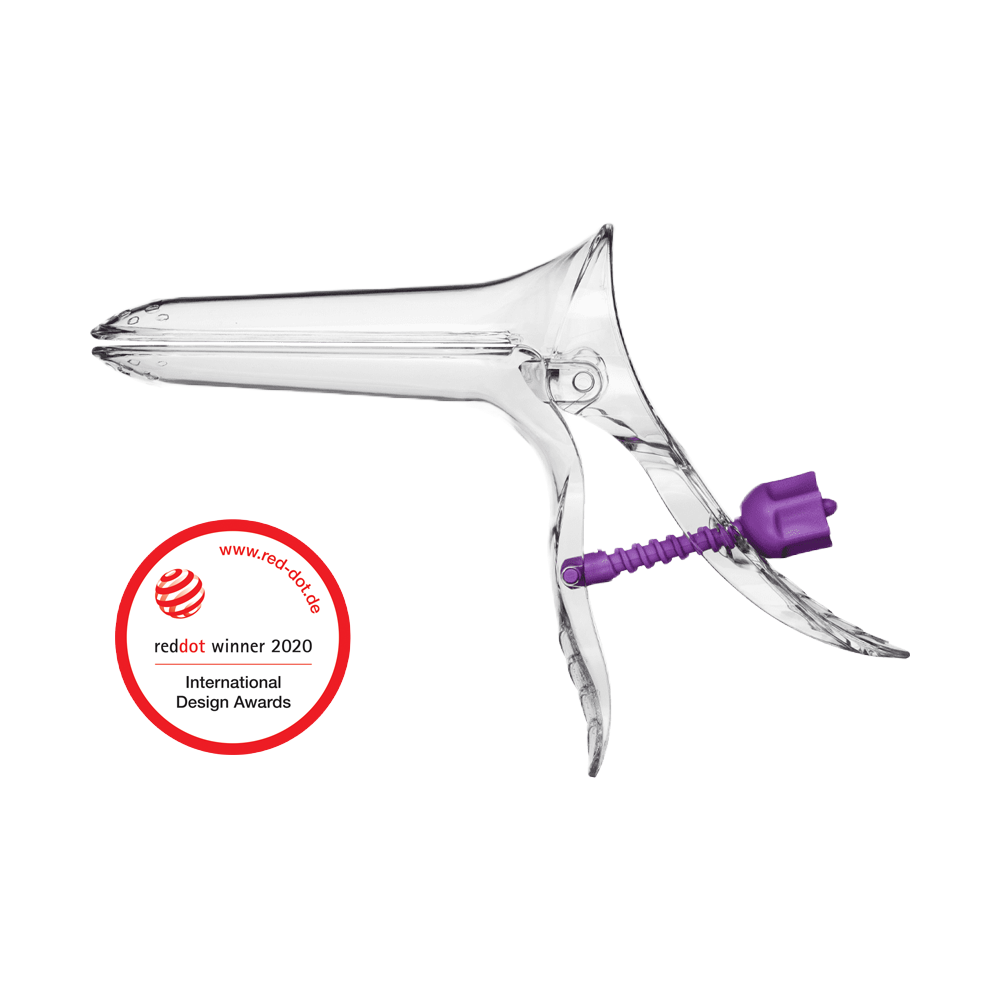
Instraspec Contour® is an award-winning, safe, unbreakable plastic single-use speculum that also delivers exceptional value for money.
Gynaecological examinations and procedures can often be daunting for patients. Both patient and clinician require a vaginal speculum that is safe and comfortable with no risk of cross-infection. Instraspec Contour® addresses these needs and more thanks to thoughful design and the use of advanced materials in its manufacture.
Why choose the Instraspec Contour®?
Class IIa medical device - audited by our notifed body
Unbreakable when in normal use - when locked can be forced down without breaking
Pivoting nut & runner providing clear visibility & access for instruments
Prestigious Red Dot Award winner
Anti-pinch hinge and beak design - for patient comfort
Gel pockets for enhanced lubrication retention
Guided nut and runner for silent and smooth operation
Wide range of sizes - five sizes to suit patients' need
100% Latex free
"Instraspec Contour has always been, and will continue to be, my go-to speculum brand"
Sarah Bolton,
Nurse Colposcopist
A complete range of speculums to cater to all patient and professional needs
Our unbreakable* speculums are available from size extra-small to large to offer the most comfortable solution for all patients.
Instraspec Contour® Extra-Small
Available in a 25 pack
Instraspec Contour® Small
Available in a 25 pack
Instraspec Contour® Medium
Available in a 25 pack
Instraspec Contour® Medium-Long
Available in a 25 pack
Instraspec Contour® Large
Available in a 25 pack






"The functionality and aesthetics of the Instraspec Contour® are designed for a high level of user-friendliness and an increase in patient acceptance"
Red Dot Design Award: Intraspec Contour®
Conformity and Standards
It is a legal requirement for all medical devices to comply with the Medical Devices Directive (MDD) 93/42 EEC and carry the CE mark. Regulatory requirements differ according to the risk attached to the use of the product. The lower the risk, the easier it is to comply with the Directive.
Single-use instruments are relatively high risk as many are surgically invasive and therefore classified as lla devices. They are therefore subject to greater levels of control than reusables, which are Class l devices except those used on central nervous tissue. Class l devices can be sold without any authorisation from an independent third party - a Notified Body. Class lla devices require a Notified Body to assess the production of the device to Annex lV, V or Vl of the Directive and issue a certificate of conformance before a CE mark can be applied and the product legally sold.
Certification by the British Standards Institution (BSI) verifies that Instraspec Contour® products meet fully the requirements of the Regulation.
The full range of unbreakable* vaginal speculums



View the full Women's Health portfolio
Looking for something else within Women's Health? We've got you covered with our extensive Women's Health portfolio.
Contact Us